In the pharmaceutical industry, a fault in manufacturing isn’t just a production flaw — it’s a potential failure in the clinical trial phase, regulatory delays, or even risks to patient safety.
When we think about clinical trials, we often imagine lab-coated scientists, sterile environments, and patients testing new treatments. But a critical aspect that often gets overlooked in the clinical trial journey is manufacturing. Manufacturing a drug is the foundational step that involves complex processes, precise formulation, and rigorous quality control. Any inconsistency, contamination, or quality lapse during the drug manufacturing phase can derail a clinical trial, no matter how well it is designed or executed.
“According to the Food and Drug Administration (FDA), 21% of clinical trial delays are attributed to manufacturing issues such as contamination, inconsistent formulation, or supply chain disruptions.”
Key Challenges Across Stakeholders in Drug Manufacturing
Before a drug ever reaches a patient in a clinical trial, it must pass through a network of tightly connected processes – from raw material procurement to quality assurance and regulatory documentation – different stakeholders. Each stakeholder along this chain faces unique operational and strategic challenges that can disrupt timelines and compromise trial readiness, such as:
Manufacturing Operations Teams
Responsible for batch production, process control, and equipment uptime, these teams often struggle with unplanned downtime, process variability, and manual error tracking. Such issues can lead to inconsistent batch quality and missed clinical supply deadlines.
Quality Assurance (QA) & Quality Control (QC) Team
Maintaining GMP compliance and product integrity, QA/QC teams are frequently challenged by slow defect detection, scattered documentation, and delayed root-cause analysis. These inefficiencies can cause regulatory delays or product holds.
Supply Chain & Logistics Managers
They are responsible for checking the timely and compliant movement of drug components and finished products. Forecasting inaccuracies, cold-chain failures, and inventory mismanagement are some challenges that they face, which can result in late deliveries of investigational drugs to trial sites.
Clinical Operations Teams
This group coordinates the flow of study materials to sites and ensures drugs meet protocol specifications. When manufacturing fails to deliver consistent formulations, it can trigger dosing errors, protocol deviations, or even force trial protocol amendments.
Regulatory & Compliance Officers
Responsible for ensuring documentation, batch traceability, and audit readiness, these stakeholders often work with fragmented data across multiple systems. This slows down approvals and exposes the organization to compliance risks during inspections.
Manufacturing Analytics: The Unsung Hero of Clinical Trial Success
When we think about clinical trials, we usually focus on research design, patient recruitment, and regulatory approvals. But there’s another critical part that often doesn’t get as much attention: making the trial drug itself. If something goes wrong in the manufacturing process, like delays, poor quality, or compliance issues, it can slow down or even ruin the entire trial.
That’s why data-driven manufacturing analytics has become indispensable. It empowers pharmaceutical teams with real-time visibility, predictive insights, and actionable intelligence on the production floor. By leveraging advanced data analytics tools, including automation, machine learning, and natural language processing, drug manufacturing for clinical trials can become more resilient, responsive, and precise.
Let’s explore eight powerful ways analytics makes a difference in drug manufacturing:
1. Ensure Consistent Drug Quality: In a clinical trial, every dose of the drug must be the same. A small change in ingredients or how it’s made could affect results. Here manufacturing analytics can play a significant role by providing visibility in key metrics such as batch-to-batch variability, deviation rate and out-of-specification events. With predictive analytics, teams use data from past and current batches to find any small changes in the process. This could be a change in temperature or in machine behavior, or slight differences in raw materials. By comparing real-time data with historical patterns, these issues can be fixed before they affect drug quality, ensuring uniformity and reliability in the trial drug.
2. Reduce Manufacturing Delays: Delays in manufacturing can hold the entire trial timeline. Whether it’s a broken piece of equipment or a late shipment of materials, the cost of waiting is high. Manufacturing analytics helps avoid these situations by identifying warning signs early. For instance, if a machine has been showing subtle signs of wear or a supplier has been slowing down, these patterns can be detected using predictive analytics before they cause delays. Production schedules become easier to plan and stick to timelines. Metrics like mean time between failures (MTBF), supplier on-time delivery rate, and production schedule adherence help ensure drug availability stays in sync with clinical milestones, avoiding bottlenecks that could set the trial back by weeks or even months.
3. Enable Real-Time Monitoring: Manufacturing analytics equipped with IoT-enabled sensors continuously track key conditions like temperature, humidity, and pressure across the production floor. These sensors send real-time data to centralized dashboards, giving teams instant visibility into the environment. If anything starts to drift from the ideal range, automated alerts go out right away. With the help of dashboards enabled by data visualization tools such as Power BI or Tableau, teams can find trends, detect issues, and take immediate action. By tracking metrics such as environmental condition deviation rate, real-time alert response time, and sensor uptime percentage, teams can ensure a stable and compliant environment that protects drug quality at every step.
4. Enhance Regulatory Compliance: world of drug manufacturing is tightly regulated, and for a good reason – lives are at stake. Keeping up with compliance requirements means having detailed, accurate records for every step of production. Manufacturing analytics enables maintaining electronic batch records with complete data lineage – raw material usage, processing parameters, quality test – which help in tracing the history of a product batch, in case of an audit. Advanced manufacturing analytics provides proactive quality control by continuously monitoring process parameters and product quality; and flagging any deviations that happen outside the approved limits. Metrics such as compliance audit pass rate, data integrity error rate, and CAPA (Corrective and Preventive Action) closure time make it easier to assess regulatory readiness and streamline audit processes.
5. Minimize Wastage and Rework: When a batch goes wrong, it wastes both materials and time. Manufacturing analytics helps spot small issues before they turn into big problems. For example, if a pattern of faulty outputs begins to appear, teams can investigate and fix it early, rather than continuing to produce unusable products. Over time, this leads to higher first-pass yield rates, lower scrap, and a smoother, more efficient process that supports the trial’s strict timelines. Tracking first-pass yield, scrap rate, and rework rate allows teams to quantify improvements and drive down losses.
6. Optimize Scale-Up Processes: When it’s time to move from small-batch production to larger manufacturing, many new challenges appear, like differences in equipment, materials, or even how long a step takes. Manufacturing analytics helps bridge this gap by comparing lab results with pilot batches and early production runs, identifying what adjustments are needed to get consistent results. Metrics such as scale-up success rate, batch consistency score, and time to commercial readiness guide teams in fine-tuning processes so they work reliably at scale, without going through multiple rounds of trial and error.
7. Improve Traceability and Audit Readiness: If something goes wrong during a clinical trial such as an unexpected side effect, it’s essential to trace exactly which batch the drug came from, how it was made, and under what conditions. Manufacturing analytics makes this kind of traceability possible by keeping a detailed, time-stamped record of every step in the process. Instead of digging through spreadsheets and logs, investigators can quickly follow the trail from ingredient to output. Metrics like lot-level traceability coverage, data retrieval time, and root cause identification rate improve the speed and accuracy of investigations and audits.
8. Support Demand Forecasting: Clinical demand is dynamic, affected by enrollment rates, patient dropouts, and site expansions. Forecasting models, powered by real-time trial data and historical usage patterns, help teams plan production more accurately. Whether demand surges at a specific site or slows down unexpectedly, teams can make timely adjustments to avoid both overproduction and stockouts. Metrics such as forecast accuracy, stockout incidence rate, and production adjustment lead time help minimize waste, reduce costs, and ensure an uninterrupted supply of the trial drug where and when it’s needed.
Trending Technologies Empowering Manufacturing Analytics
Modern pharmaceutical manufacturing is undergoing a digital transformation driven by Industry 4.0 principles. These technologies bring intelligence, automation, and precision to every stage of production and make the entire drug development process more efficient, compliant, and clinical trial ready. Below are some of the key technologies enabling this transformation in clinical manufacturing:
1. AI and Machine Learning (ML)
AI and ML play an important role in manufacturing analytics by helping teams make sense of large volumes of fast-moving data coming from equipment sensors, process logs, and quality reports. These technologies can find complex patterns and connections that are hard to spot with traditional analysis, allowing for quicker, smarter decisions on the production floor.
Key Applications
- Batch Failure Prediction: By using machine learning models to analyze historical production data, manufacturers can discover which factors like machine temperature, shift timing, or specific material lots are most often linked to batch failures or deviations. These predictive insights help teams take early action to avoid producing batches that fall outside of quality standards.
- Contamination Risk Detection: AI systems can continuously track environmental data such as air quality, cleanroom pressure, and raw material conditions. If the system finds something unusual like a drop in air purity or a change in raw material quality, it can send early alerts through real-time analytics dashboards. These warnings help prevent contamination, reduce the risk of batch loss, and keep clinical trials on schedule and in compliance.
2. IoT and Smart Sensors
The Internet of Things (IoT) connects manufacturing equipment and cleanroom environments through smart sensors that send data continuously. This constant flow of information feeds directly into manufacturing analytics systems, helping teams monitor conditions in real time and respond quickly when needed.
Key Applications
- Environmental Monitoring: Sensors collect real-time data on temperature, humidity, and pressure, critical factors for ensuring drug stability and sterility, especially for sensitive products like biologics and injectables. This data is visualized through analytics dashboards, allowing teams to track trends and maintain control.
- Automated Alerts and Logs: When any reading goes beyond its acceptable range, this system immediately sends alerts and logs the event with a timestamp. These logs are not only valuable for quick action but also for audit trails and quality investigations.
3. Digital Twins
A digital twin is a virtual model of a real-world manufacturing process, built using real-time and historical data. It allows teams to simulate, analyze, and improve operations without disrupting actual production. Connected to analytics platforms, digital twins turn raw data into valuable insights for process optimization.
Key Applications
- Process Simulation: Before moving a process from lab to large-scale production, teams can run simulations to test different settings like temperature, mixing speeds, or ingredient ratios. Digital twins help evaluate outcomes and identify the best combination for consistent results.
- Formulation Scaling: Digital twins reduce the need for trial-and-error during scale-up. By analyzing data from both R&D and early production runs, they guide adjustments that minimize variability and improve quality across batches.
4. Cloud-Based Analytics Platforms
Cloud platforms centralize all data, such as production, quality, supply chain, and maintenance, into a unified, accessible, and scalable system.
Key Applications
- Cross-Site Visibility: With manufacturing spread across geographies, cloud platforms provide a single view of operations, enabling better coordination among teams at different plants, contract manufacturers (CMOs), and trial sponsors.
- Real-Time Collaboration: Cloud-based dashboards and analytics tools allow stakeholders (QA, operations, regulatory, and clinical) to access insights from anywhere, accelerating decision-making and issue resolution.
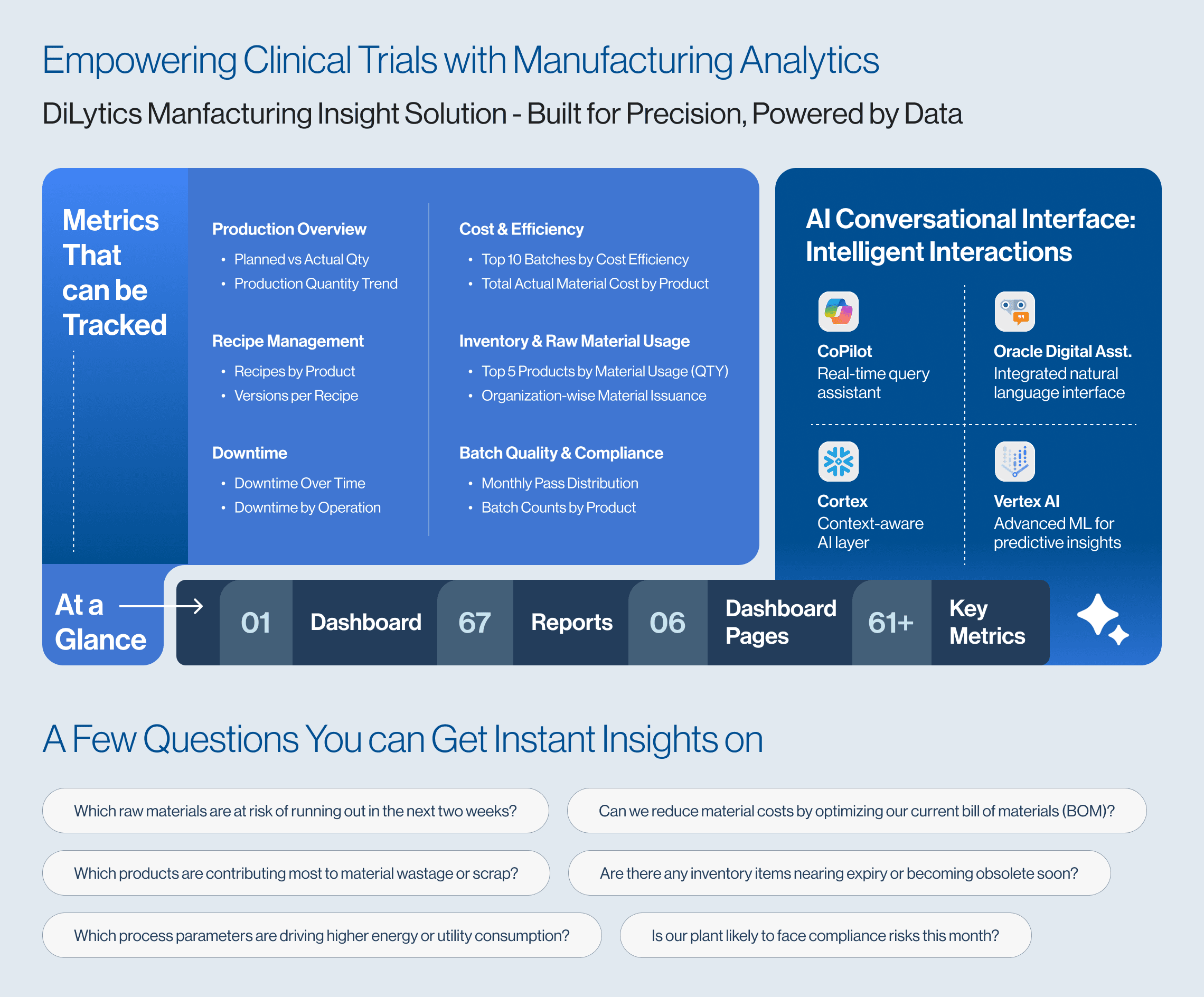
Make Clinical Trials Smarter with DiLytics Manufacturing Insight Solution
At DiLytics, we understand that clinical trials depend not only on scientific innovation but also on manufacturing precision. That’s why we bring deep expertise in data analytics to help pharmaceutical companies transform data into actionable insights that drive consistency, quality, and compliance in the manufacturing process.
To ensure your clinical trials are built on a foundation of consistent, compliant, and high-quality drug manufacturing, DiLytics offers the Manufacturing Insight Solution – a powerful, pre-built analytics platform tailored for pharmaceutical and life sciences industries. Equipped with pre-packaged business metrics, dashboards, reports, and KPIs, it integrates seamlessly with ERP and MES systems to transform raw shop-floor data into actionable intelligence. With this solution, you can:
- Predict and plan smarter by simulating production outcomes based on fluctuating demand or material delays
- Gain tighter control over material costs by identifying high-cost components and uncovering opportunities for optimization
- Stay informed with real-time inventory visibility, including expiry alerts and wastage tracking
- Gain full visibility into production progress, exceptions, and timelines with batch-level insights
Success Story: Insights to Spot Business Process Improvement Opportunities
A global biotech company specializing in rare genetic therapies struggled with limited visibility into key manufacturing data, leading to inefficiencies and delays in decision-making. DiLytics implemented Oracle Manufacturing Analytics, integrating data from Oracle E-Business Suite (EBS) and Advanced Supply Chain Planning (ASCP). The solution delivered real-time insights into production, materials, and inventory, enabling faster decision-making, streamlined reporting, and proactive issue management. This empowered the manufacturing team to identify bottlenecks, optimize processes, and improve overall efficiency.
To read the full story, click here.
Final Thoughts: Manufacturing Intelligence Is the Bridge to Better Clinical Outcomes
Clinical trials demand precision and consistency, even small changes in ingredients, processes, or equipment can lead to delays, higher costs, or risks to patient safety. This is where manufacturing analytics makes a big difference. By turning complex production data into clear insights, analytics helps pharma companies find issues early, prevent deviations, and stay aligned with regulations. Instead of reacting after problems occur, teams can predict, prevent, and optimize, moving medicines from lab to patient faster and more reliably.
With a pre-built solution like DiLytics Manufacturing Insight, this becomes even easier. Instead of spending months creating custom tools, pharma teams can instantly use ready-made dashboards built specifically for pharmaceutical manufacturing. These dashboards provide visibility into batch consistency, yield, cycle times, and quality metrics, all while following industry best practices and regulatory standards. The result? Streamlined operations, data-backed decisions, and a stronger compliance foundation.
Ready to explore how DiLytics can support your clinical and operational goals? Let’s connect for a quick conversation.
Frequently Asked Questions (FAQs)
Q1: Why is manufacturing analytics important before a clinical trial?
Answer: Clinical trials rely on consistent, high-quality drug formulations. Manufacturing analytics helps ensure that every batch meets the required quality standards, reducing the risk of delays, regulatory issues, or patient safety concerns.
Q2: How does real-time monitoring impact clinical trial success?
Answer: Real-time monitoring provides immediate visibility into production conditions, helping detect deviations early. This ensures drugs used in trials are produced under optimal conditions and remain stable and effective.
Q3: Can small biotech firms benefit from manufacturing analytics?
Answer: Absolutely. Manufacturing analytics solutions can be tailored to fit the needs of startups and mid-sized companies, providing the same quality and compliance benefits without enterprise-level complexity.
Q4: How does manufacturing analytics support regulatory compliance?
Answer: It automates data capture, tracks batch histories, and maintains audit trails, making it easier to comply with FDA, EMA, and GxP standards.
Q5: What role do digital twins play in clinical manufacturing?
Answer: Digital twins simulate manufacturing processes to identify the best production parameters before scaling up. This improves formulation consistency and reduces the risk of trial failure.




